When in the course of human events it becomes necessary for patients to dissolve the chemical bonds which have connected them with opioids, one can look to the Centers for Disease Control for guidance.
Tapering opioids (and benzodiazepines) was addressed multiple times in the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain. Here are some instructive excerpts, reproduced in an effort to preserve consistency with the guideline’s overriding message…

Link: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm
Established patients already taking high dosages of opioids, as well as patients transferring from other clinicians, might consider the possibility of opioid dosage reduction to be anxiety-provoking, and tapering opioids can be especially challenging after years on high dosages because of physical and psychological dependence.
Experts noted that patients tapering opioids after taking them for years might require very slow opioid tapers as well as pauses in the taper to allow gradual accommodation to lower opioid dosages.
Clinicians should remain alert to signs of anxiety, depression, and opioid use disorder that might be unmasked by an opioid taper and arrange for management of these co-morbidities.
For patients agreeing to taper to lower opioid dosages as well as for those remaining on high opioid dosages, clinicians should establish goals with the patient for continued opioid therapy, maximize pain treatment with nonpharmacologic and nonopioid pharmacologic treatments as appropriate, and consider consulting a pain specialist as needed to assist with pain management.
Although the clinical evidence review did not find high-quality studies comparing the effectiveness of different tapering protocols for use when opioid dosage is reduced or opioids are discontinued, tapers reducing weekly dosage by 10%–50% of the original dosage have been recommended by other clinical guidelines, and a rapid taper over 2–3 weeks has been recommended in the case of a severe adverse event such as overdose.
Experts noted that tapers slower than 10% per week (e.g., 10% per month) also might be appropriate and better tolerated than more rapid tapers, particularly when patients have been taking opioids for longer durations (e.g., for years).
Opioid withdrawal during pregnancy has been associated with spontaneous abortion and premature labor.
When opioids are reduced or discontinued, a taper slow enough to minimize symptoms and signs of opioid withdrawal (e.g., drug craving, anxiety, insomnia, abdominal pain, vomiting, diarrhea, diaphoresis, mydriasis, tremor, tachycardia, or piloerection) should be used.
A decrease of 10% of the original dose per week is a reasonable starting point; experts agreed that tapering plans may be individualized based on patient goals and concerns.
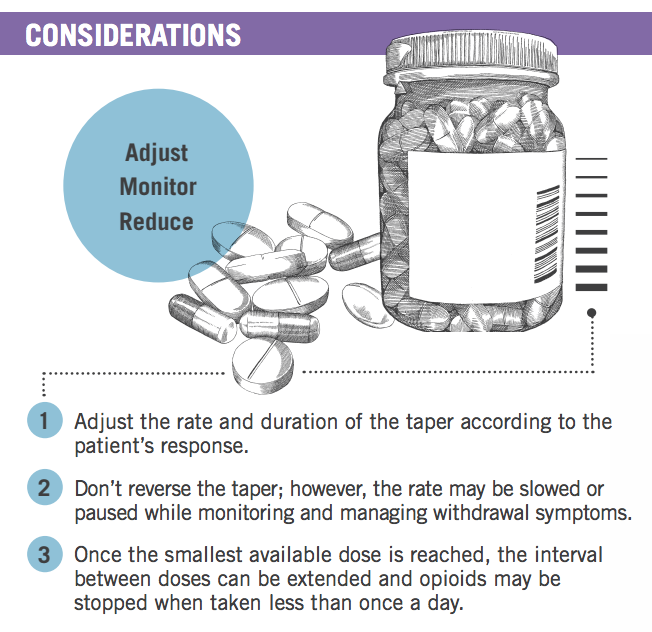
Experts noted that at times, tapers might have to be paused and restarted again when the patient is ready and might have to be slowed once patients reach low dosages.
Tapers may be considered successful as long as the patient is making progress. Once the smallest available dose is reached, the interval between doses can be extended. Opioids may be stopped when taken less frequently than once a day.
More rapid tapers might be needed for patient safety under certain circumstances (e.g., for patients who have experienced overdose on their current dosage). Ultrarapid detoxification under anesthesia is associated with substantial risks, including death, and should not be used.
Clinicians should access appropriate expertise if considering tapering opioids during pregnancy because of possible risk to the pregnant patient and to the fetus if the patient goes into withdrawal.
Patients who are not taking opioids (including patients who are diverting all opioids they obtain) do not require tapers.
Clinicians should discuss with patients undergoing tapering the increased risk for overdose on abrupt return to a previously prescribed higher dose.
Primary care clinicians should collaborate with mental health providers and with other specialists as needed to optimize nonopioid pain management, as well as psychosocial support for anxiety related to the taper.
If a patient exhibits signs of opioid use disorder, clinicians should offer or arrange for treatment of opioid use disorder and consider offering naloxone for overdose prevention.
For pregnant women already receiving opioids, clinicians should access appropriate expertise if considering tapering opioids because of possible risk to the pregnant patient and to the fetus if the patient goes into withdrawal.
If tests for prescribed opioids are repeatedly negative, confirming that the patient is not taking the prescribed opioid, clinicians can discontinue the prescription without a taper.
Because of greater risks of benzodiazepine withdrawal relative to opioid withdrawal, and because tapering opioids can be associated with anxiety, when patients receiving both benzodiazepines and opioids require tapering to reduce risk for fatal respiratory depression, it might be safer and more practical to taper opioids first.
Clinicians should taper benzodiazepines gradually if discontinued because abrupt withdrawal can be associated with rebound anxiety, hallucinations, seizures, delirium tremens, and, in rare cases, death.
A commonly used tapering schedule that has been used safely and with moderate success is a reduction of the benzodiazepine dose by 25% every 1–2 weeks.
If benzodiazepines prescribed for anxiety are tapered or discontinued, or if patients receiving opioids require treatment for anxiety, evidence-based psychotherapies (e.g., CBT) and/or specific anti-depressants or other nonbenzodiazepine medications approved for anxiety should be offered. Experts emphasized that clinicians should communicate with mental health professionals managing the patient to discuss the patient’s needs, prioritize patient goals, weigh risks of concurrent benzodiazepine and opioid exposure, and coordinate care.
For patients with problematic opioid use that does not meet criteria for opioid use disorder, experts noted that clinicians can offer to taper and discontinue opioids. For patients who choose to but are unable to taper, clinicians may reassess for opioid use disorder and offer opioid agonist therapy if criteria are met.

https://www.cdc.gov/drugoverdose/pdf/Clinical_Pocket_Guide_Tapering-a.pdf










CDC Guideline for Prescribing Opioids for Chronic Pain www.cdc.gov/drugoverdose/prescribing/guideline.html
Washington State Opioid Taper Plan Calculator www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf
Tapering Long-Term Opioid Therapy in Chronic Noncancer Pain www.mayoclinicproceedings.org/article/S0025-6196(15)00303-1/fulltext